Lab prova
Your Reliable Partner | Laboratory for Validation and Qualification | Professional Measuring Equipment
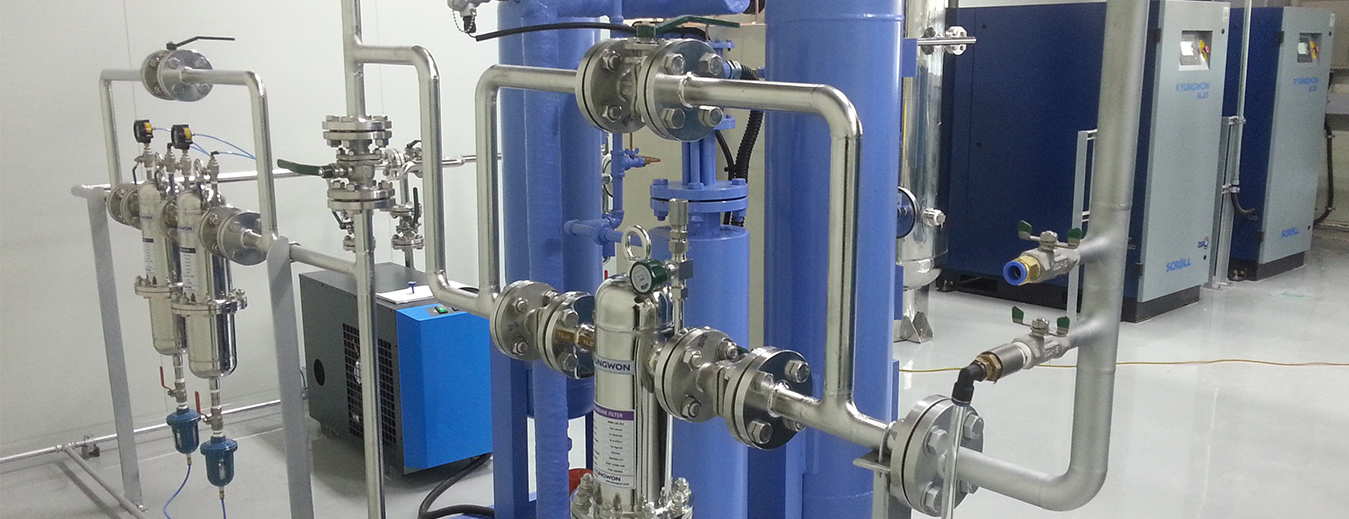
Identifying difference between critical and non-critical parameters play
an important role in the quality risk management process. The parameters
which may affect product quality need to be identified as critical,
clearly documented and be shown to be maintained within requirements.
Compressed air in most of the GMP production processes come into
direct contact with the product, and as such needs to be identified as
critical utility the variability of which has an impact on the product
quality and therefore should be monitored or controlled.
Compressed air should be dry, clean and oil free and the quality should be confirmed.
We are able to provide testing of compressed air contaminants and purity classes, including critical parameters such as:
Qualification of compressed air (and other process or laboratory gases) in the pharmaceutical industry need to be followed by appropriate VALIDATION AND QUALIFICATION DOCUMENTATION.






LAB PROVA was founded with the aim to fulfill the need of the local market for laboratory that would be able to provide professional cleanroom validation service. Specific needs of the pharmaceutical industry, through the years, naturally shaped our professional approach, dedicated to customer satisfaction.
Knowledge about applications, experience, research and development helped us to establish strong co-operation with world leading manufacturers of measuring equipment, for a wide range of industrial applications.